Talk the talk and walk the walk!
Using machine learning to identify adverse events in scientific biomedical literature
Recently we have often mentioned how Averbis Information Discovery can play a significant role in improving your pharmacovigilance and adverse event detection efforts. Today we want to show you an example from a joint publication with Bayer AG’s Sonja Wewering, Claudia Pietsch and Anna-Theresa Lülf-Averhoff and our Marc Sumner, Kornél Markó, and David Baehrens (Full article linked below).
In this peer-reviewed study, it was shown how machine learning can be used to reduce noise and automatically pre-classify large sets of documents for expert review. By using the available historic training data and expert knowledge, Information Discovery into their workflow to combine automated and intellectual reviews to reach near 100% correctness.

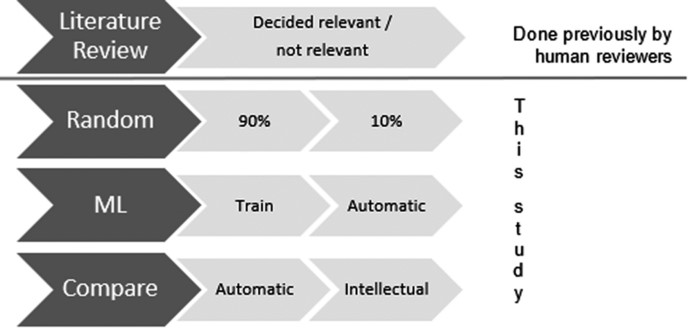
These results do not only show the significant benefits machine learning holds for the future of literature reviews but also show how through this we can improve patients’ lives by missing no relevant adverse events for further research.
Full article:
Wewering S, Pietsch C, Sumner M, Markó K, Lülf- Averhoff A- T, Baehrens D. Machine learning approach to identify adverse events in scientific biomedical literature. Clin Transl Sci. 2022;00:1– 7. doi: 10.1111/cts.13268
https://ascpt.onlinelibrary.wiley.com/share/2UWZ4MVDA5BCMRFUR6ER?target=10.1111/cts.13268
Benefit from state-of-the-art AI
Do you want to reap the same benefits for your organization?
Get in touch today and we will show you how you can benefit from machine learning in your pharmacovigilance processes!

