IDMP Text Mining
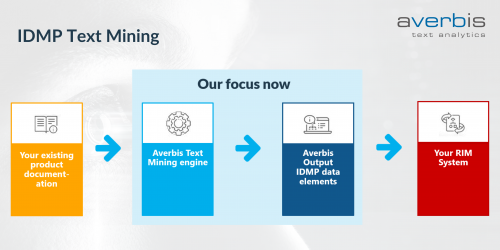
With the introduction of the IDMP standards many companies now face the challenge to accurately and cost effectively collect relevant data for their regulatory affairs. Traditionally, this constitutes a mainly manual task of going through thousands of documents to extract the needed information. The result often is a time intensive and expensive process that is exposed to human errors.
- Fully automate IDMP data extraction with NLP
- Custom IDMP libraries and annotators
- Multilingual terminology management
- Controlled vocabularies maintained by the EMA
- Easy to integrate into your existing systems with open API’s
This allows you to:
Partners
We have partnered with Amplexor Life Sciences to integrate our IDMP text mining solution into their Life Sciences Suite, an IDMP compliant RIM solution. This provides the perfect solution if you don’t have a regulatory information management solution yet but still want to benefit from automated IDMP data extraction. To find out more about the partnership and Amplexor click here.

Related Information
Scientific Publication (Regulatory Intelligence)

To find out more on text mining for regulatory intelligence check out the following publication (“Text mining for regulatory intelligence: taking an automated approach”) by our esteemed colleagues Harsha Gurulingappa (Text Analytics Product Owner, IT Advanced Analytics), Dominik Schneider (Senior Architect, IT Advanced Analytics), Moritz Kloft (Senior IT Project Manager, IT Healthcare) from Merck KGaA, Darmstadt, Germany; and Janaki Suriyanarayanan (Senior Manager – Regulatory Information Management), Joerg Werner (Associate Director – Regulatory Data Governance) from the Global Regulatory Affairs team at Merck Healthcare KGaA. To get to the full publication click here.
Customer Voice
“We had a successful PoC of the Averbis IDMP Pipeline.
At the beginning of the PoC we were a bit nervous because of the heterogeneity of our documents. But the quality of the extraction of the existing data elements was good out of the box and could be increased to the required level by small pipeline adjustments to our documents. For our additional required data elements, it was shown that they could be successfully added in a short time. The obvious flexibility of the Averbis solution convinced us here. We were also able to use the extracted data in our systems with little effort during the PoC.
During the PoC, it occurred to us that we could also use the address harmonization (module 3 extraction) for other use cases in production. That was a nice side effect!
Once again, we would like to especially emphasize the determined and pleasant cooperation with the Averbis project team.”
Director/Senior Manager Process & QM IT of large pharmaceutical company (~10 billion sales)