AI assisted Pharmacovigilance and Adverse Event Management
More time for patient safety!
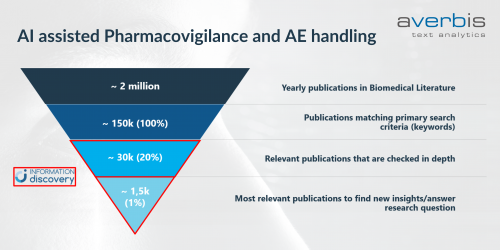
Every year, millions of Biomedical publications are published of which pharmaceutical companies want to find the 1,5k – 30k articles relevant to their pharmacovigilance efforts. With the traditional approach using keyword indexing these millions of articles can be cut down to a set of approximately 150k potentially relevant articles, which then have to be evaluated and classified manually to get to the 1,5k – 30k really relevant ones. This does not only produce a huge manual labor burden on pharmacovigilance departments but also costs them valuable money and time.
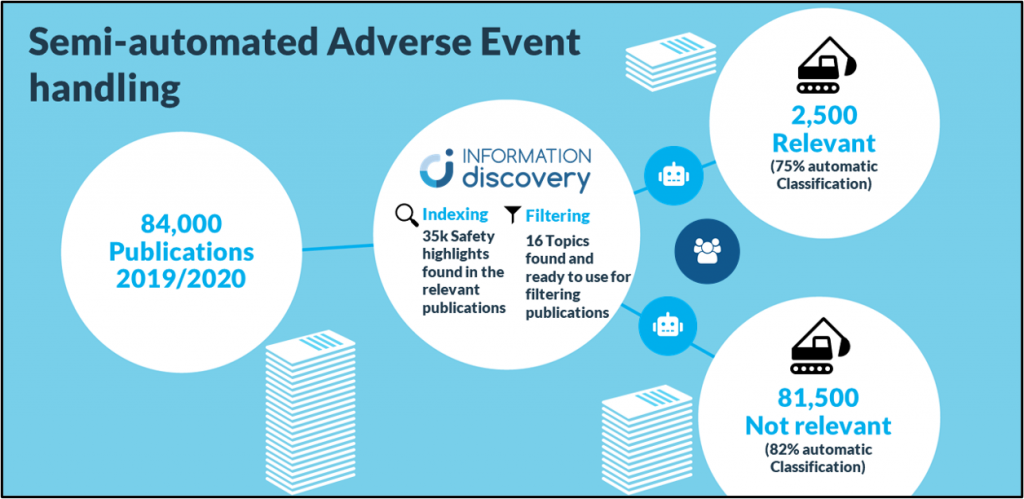
Using a combination of artificial intelligence and natural language processing, Averbis Information Discovery can help you get those 1,5k -30k relevant articles needing only 20% of your original workload. Information Discovery can be connected directly to your or any public data lake through API’s and import the documents selected for screening. Then the system automatically classifies, categorizes and indexes these documents using a machine learning algorithm trained on your decision making.
The results can then be exported to your existing systems or into your existing workflow. This way you not only get the necessary results for pharmacovigilance reporting, but also gain additional insights from the literature screened which traditionally would have been lost. Therefore, Information Discovery does not only lower your workload and associated costs but also provides you with more accurate results and new insights.
This allows you to:
Resources
Success Story
Check out the success story below to find out how we validated this at a major pharma company in Germany. (Click image to download)
Scientific Publication
Check out this scientific publication to see how we automated the pre-selection of PV relevant documents containing adverse events using machine learning. (Click image to download)