
- Averbis Health Discovery enables reliable de-identification of medical documents in accordance with the HIPAA Safe Harbor method.
- Sensitive patient information remain protected.
- Patient information can be used for medical research, quality assurance and clinical studies in a privacy-compliant manner.
Secondary use of routine medical data in research
The provision of clinical raw data is an indispensable basis for numerous applications in medical research: e.g. aggregated patient data can help to identify disease mechanisms, reduce recruitment times of patients in clinical studies or improve medication safety monitoring. However, such projects often fail because the raw data contain personal information and there is no way to quickly and reliably deidentify large volumes of data. This is exactly where Averbis Health Discovery can help.
High need for protection of clinical data
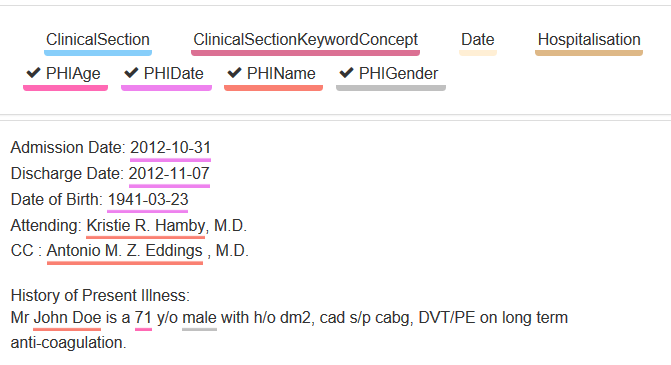
Personal medical data is highly sensitive and subject to strict data protection regulations. All personal information must be removed before the data can be released for medical research. In addition to personal names and dates of birth, telephone numbers, names of medical staff and relatives, many other text passages are also part of the information that needs to be protected. Depending on the application, information such as dates should not be completely removed, but should be coarsened to one or more years for specific studies.
Best possible protection through a combination of AI and pattern recognition
Averbis Health Discovery supports you in the de-identification of personal data in medical free texts in compliance with HIPAA. In order to ensure the best possible protection of data, current technologies from the field of deep learning are combined with pattern-based procedures: for example, names, location information, occupational information and other features are recognized by means of artificial intelligence, the identification of e-mail address and date information is additionally secured by means of pattern recognition.
Individually adaptable to your needs
The marking of personal data and their further processing are logically separated in Averbis Health Discovery. Thus, you have the possibility to treat the identified characteristics differently according to requirements. You would like to obtain the patient’s year of birth in one study, and completely remove the year in another study? The de-identification is flexibly adaptable and provides reliable protection in every case of application.
De-identification fast and safe
The de-identification of Averbis Health Discovery is an indispensable tool for distributed medical research and finds its application wherever personal information is to be protected. It supports the data protection-compatible handling of medical documents in clinical studies, quality assurance and medical research. The de-identification as well as all other modules of Averbis Health Discovery are available for different languages.

