IDMP – IDENTIFICATION OF MEDICINAL PRODUCTS
ISO IDMP ist eine Sammlung von ISO Standards, welche die Basis für eine eindeutige einheitliche Identifikation medizinischer Produkte bilden
Ab Juli 2016 müssen alle pharmazeutische Unternehmen, die Medikamente in Europa zugelassen haben, die neuen ISO-Standards einhalten.
Die Standards benötigen Informationen aus einer Vielzahl pharmazeutischer Bereiche, einschließlich Zulassung, Forschung und Entwicklung, Herstellung und Vertrieb.




WER IST VON IDMP BETROFFEN?
- Mitarbeiter der Zulassungsabteilungen pharmazeutischer Unternehmen
- IT-Abteilungen der Arzneimittelbehörden, der pharmazeutischen Unternehmen und der Dienstleister
- Qualifizierte Personen der EU, der Zulassungsbehörden und pharmazeutischer Unternehmen im Bereich Pharmakovigilanz
- Softwareanbieter für das Management medizinischer Produkte
- Sponsoren klinischer Studien
HERAUSFORDERUNGEN BEI DER IMDP-UMSETZUNG
- Unternehmen müssen sich mit der Komplexität der IDMP relevanten Daten und ihrer Organisation auseinander setzen
- IDMP relevante Informationen können in verschiedenen Quellsystemen verstreut sein, die die Daten typischerweise in sehr heterogener Form enthalten
- Die Mehrheit der IDMP relevanten Daten liegen in unstrukturierter Form vor, z.B.
- Zusammenfassung der Merkmale des Arzneimittels
- Pharmazeutische Dokumente / CPC Dokumente
STRUCTURE THE UNSTRUCTURED
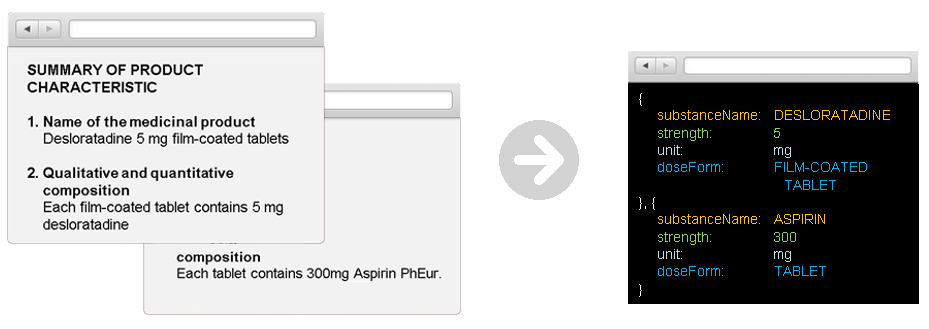
Information Discovery ermöglicht die Strukturierung von Informationen, die in unterschiedlichsten IDMP relevanten Quell-Dokumenten enthalten sind
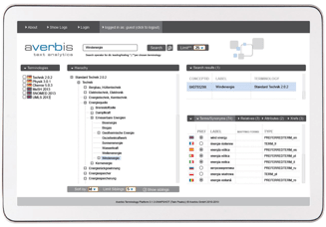
KONTROLLIERTE VOKABULARIEN
- Kontraindikationen
- Krankheits-Status
- Geschlecht
- Indikationen
- Symptome
- Unerwünschte Wirkungen
Information Discovery beinhaltet ein mehrsprachiges Terminologie-Management-System, welches die IDMP relevanten kontrollierten Vokabularien zur Verfügung stellt.
Finden Sie Antworten in Ihren Daten
Gerne präsentieren wir Ihnen unsere Produkte und erstellen für Sie einen Demonstrator auf Basis Ihrer ausgewählten Datenbestände.

